
There’s one thing that’s always certain to happen and that’s… change! You expect a “normal” day, and something happens which makes you change direction… change what you need to do next and sometimes stop what you planned to do that day altogether! In the world of manufacturing, change is a constant which affects the performance of a company, and we need to be able to plan, implement, monitor and review to make sure Safety, Quality and Efficiency are achieved! Companies which have effective Change Management are the most committed to compliance with ISO 9001 & IATF 16949 and have the means to manage change, expected and unexpected.
Change – what is it?
As a verb, change can be defined as “make (someone or something) different; alter or modify”.
Or
As a noun “an act or process through which something becomes different”.
In automotive manufacturing, change is something which continuously happens. Automotive manufacturers constantly work towards improved products and innovations to be able to stay “ahead of the curve” and compete for market share.
When an idea or proposal is formed, it must be studied, reviewed and managed before full implementation. How many times have you seen a poorly thought-out idea go wrong and cost time, money or your reputation?
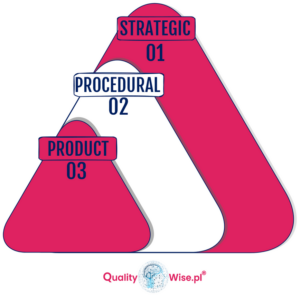
Types of changes in change management – what are they?
Strategic type in change management
This type of change is centered around the organization’s needs to be able to achieve goals, respond to market directional changes or follow a Strength, Weakness, Opportunities and Threat (SWOT) type of analysis. Top Management typically develops this with their leadership team.
Procedural in change management
This type of change affects the way we do our work, it centers around the way we manage a task as an individual, a team or a department.
Product in change management
This type of change involves altering the material, appearance, function or shape.
Change management and the change curve
Emotions can run high when any change initiative is started, we as humans, for the most part, do not like change. We prefer our routines; anything that can disrupt this can affect us emotionally. It is important to consider how a change is communicated and who is involved in the development of the change. It would be naive to believe that only Strategic and Procedural changes can trigger us emotionally, the same can be said for Product changes.
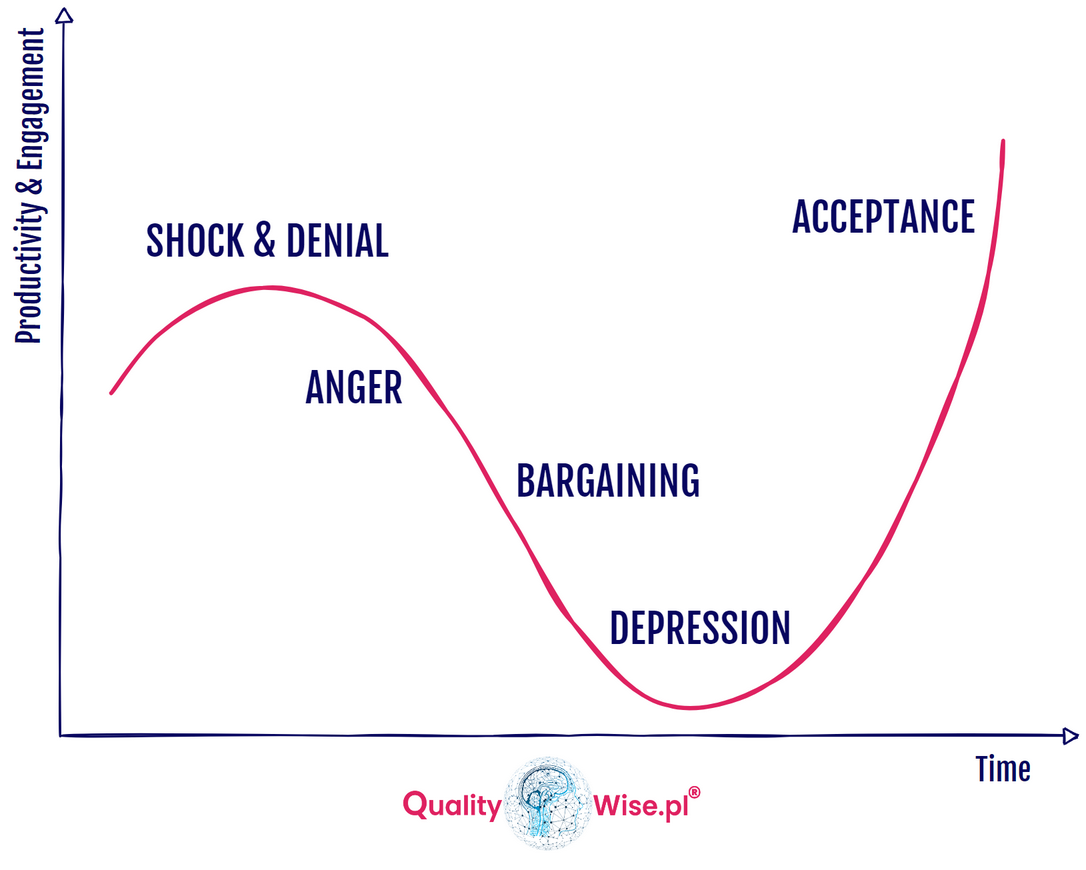
The Kübler-Ross Change Curve depicts our emotions as we process change over time, our engagement and productivity drop before eventual recovery.
Change Management as a Process
IATF 16949 in point 8.5.6.1. for the automotive industry requires the establishment of a documented process for monitoring and responding to changes that affect the implementation of the product.
Below is an example of a change management process for a product.
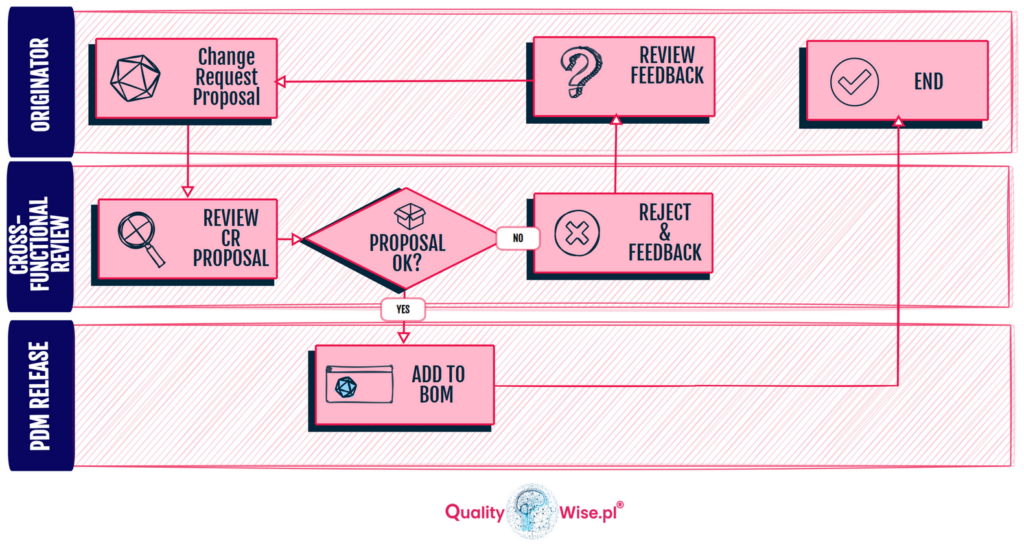
Change Request Proposal
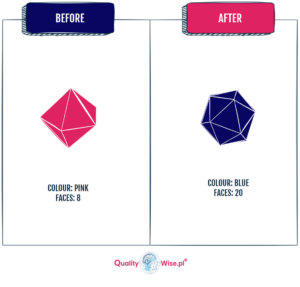
It’s important to be concise about what the change is and why the change needs to be initiated.
A good technique is to use a “before & after” approach to illustrate what the current condition is (before) and what the product will look like after.
Example:
Cross-Functional Review
Many changes fail to be effective because they are not reviewed cross-functionally within an organization.
Questions to ask:
What issue does this fix?
How urgently does this need to be processed?
Are the surrounding components in specification?
Who will make the change (internal or supplier)?
Tips for Implementing Change Management
1. Involve stakeholders: ISO 9001 clause 8.3.3 Design and Development Inputs says that you should identify the following requirements:
- Functionality;
- Performance;
- Statutory or Regulations;
- Applicable Standards;
- Similar design and development activities previously undertaken;
- Consequences of failure or risk level with the potential of failure.
Therefore, ensure changes are reviewed with input from relevant departments to capture all applicable requirements at the beginning of the change review process.
2. Involve stakeholders: ISO 9001 clause 8.3.6 Design and Development says that you should identify, review and control changes.
- what is to be done;
- what resources are needed;
- who will be responsible;
- when it will be completed;
- how to evaluate the results;
- what actions were taken to reduce risk and adverse effects due to the change.
3. Control the Change: ISO 9001 clause 8.5.6 Control of Changes stipulates that you should have a suitable process in place to be able to:
- review the change;
- if acceptable, approve the change;
- communicate the change;
- validate the change.
Where the change is not fully effective, a CAPA approach may be required to prevent issues.
Change Management in IATF 16949:2016
It’s important to understand the link between Control of Changes and IATF 16949.
Point 8.3.6.1 Changes in design and development – supplement refers to the assessment of changes in terms of their impact on:
- fit,
- form,
- functions,
- performance and/or durability.
These changes should be validated against customer requirements and internally approved before implementation into production. However, remember that nothing can be implemented without obtaining documented approval from the customer or documented consent to a deviation.
Note: we will also find a requirement here for products with embedded software. The level of software and hardware changes must be documented.
In turn, the already mentioned point 8.5.6.1 specifies that the organization should:
- define activities related to verification and validation to ensure
- compliance with customer requirements;
- validate changes before their implementation;
- document evidence related to risk analysis;
- maintain records of verification and validation.
IATF recommends conducting a production trial, necessary to verify changes, in order to assess their impact on the manufacturing process.
If the customer requires it from us, the organization should:
- inform them of any planned change in the implementation of the product since its last approval;
- obtain documented customer approval before implementing the change, and this is where the connection to PPAP comes in;
- meet additional requirements for verification or identification, such as production trial and validation of the new product.
In IATF 16949, we also have a requirement for appropriate change management for safety-related products in point 4.4.1.2. But here we invite you to explore the topic yourself.
Let’s sum up!
Reviewing change proposals cross-functionally, confirming all relevant specifications and requirements and “looking back” at similar designs can prevent adverse effects on your manufacturing processes and customers.
All change proposals must be scrutinized to understand if the change is needed, meets safety and quality objectives and does not add excessive costs to the product Bill of Materials.
Many companies struggle to manage change effectively which leads to poor Quality Performance! We hope that with this article you will avoid it in your organization.
Do you want to know more?
We invite you to the ISO 9001 and IATF 16949 organized by Qualitywise, where you will learn in detail all the requirements for the quality management system. Ask about the date.

Hope you found the article interesting.
Hope you found this article interesting. If you wish to receive our articles directly to your mailbox sign up to the newsletter!
Thank you for your presence.
For people who want to know more:
Knowledge must have a solid foundation in order to avoid information noise. Therefore, the article was based on the following literature:
IATF 16949: 2016 Requirements for quality management systems in serial production and the production of spare parts in the automotive industry, 1st edition, 2016
All content on the qualitywise.pl website is a private interpretation of publicly available information. Any convergence of the described situations with people, organizations, companies is accidental. The content presented on the website qualitywise.pl does not represent the views of any companies or institutions.
